Structure & Reactivity
Infrared Spectroscopy
IR7. Bonds to Common Heteroatoms: Nitrogen
The IR spectra of nitrogen-containing compounds can be messier than the ones you have seen so far. N-H bends and C-N stretches tend to be broader and weaker than peaks involving oxygen atoms. However, some peaks in nitrogen compounds are useful. The problems in this section will guide you through some of these features.
Problem IR7.1.
Amines, such as butylamine, have a very diagnostic N-H peak, although the peak is sometimes weak.
![]()
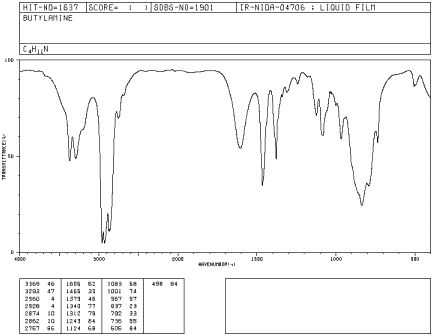
Figure IR7.1. IR spectrum of butylamine.1
Butylamine is a primary amine, meaning the nitrogen is attached to one carbon group and two hydrogens. An N-H bond is almost as strong as an O-H bond. Can you identify the N-H peak in this spectrum?
Problem IR7.2.
Dibutylamine is another example of an amine. It is a secondary amine, meaning the nitrogen is attached to one hydrogen and two other groups.
![]()

Figure IR7.1. IR spectrum of dibutylamine.1
Compare its N-H peak to that of butylamine. What do you notice about the number of N-H peaks in each spectrum?
Problem IR7.3.
What peaks would you expect to see in the IR spectrum of triisobutylamine?
Problem IR7.4.
Benzonitrile has no N-H bonds but it does have a carbon-nitrogen triple bond that shows up in the IR spectrum. Identify the corresponding peak on the spectrum, plus two other peaks.
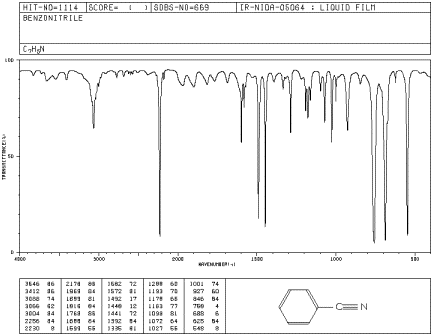
Figure IR7.3. IR spectrum of benzonitrile.1
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: